With the US and Europe prioritizing vaccine access for their own citizens, China could be the main source of vaccines for the developing world through at least early 2021. China's success in controlling Covid-19 through aggressive test-and-trace policies increases Beijing's flexibility to deploy vaccines for diplomatic purposes. China's vaccine diplomacy highlights a growing divide between developing and developed countries in terms of China's international standing.
Even as the pandemic has soured China's relationships with many wealthy democracies, China is poised to be the primary source of vaccines for developing countries through at least the first quarter of 2021, strengthening Beijing's international influence.
With supplies from Western firms running tight, many governments could turn to China. A senior Chinese government scientist said on 4 December that China will have 600mn doses of a coronavirus vaccine ready for market by year's end. By contrast, the WHO said the same day that COVAX, a multilateral initiative that aims to promote equitable access to vaccines, will have 500mn doses available around the end of March but that most distribution would not occur until the second quarter.
China's success in controlling Covid-19 through aggressive test-and-trace policies increases Beijing's flexibility to deploy vaccines for diplomatic purposes. The Chinese government has signaled its intention to distribute vaccines abroad even before most Chinese citizens have received one. In May, President Xi Jinping told the WHO that China would share vaccines as a "global public good." In June, Xi told African leaders that their countries would be "among the first to benefit" once vaccines were available. In July, Beijing pledged a USD 1bn loan to Latin America and the Caribbean to help them purchase vaccines. In August, Xi promised priority access to Cambodia, Laos, Thailand, Myanmar, and Vietnam. In October, China officially joined COVAX, while the US has declined to participate.
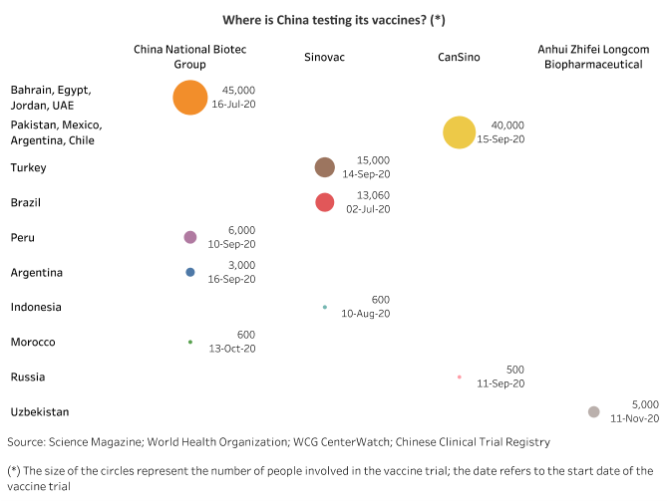
Five vaccine candidates from four Chinese companies are currently in phase 3 clinical trials in at least 15 countries, and early distribution has already begun. Last week Indonesia and Brazil received 1.2mn and 1mn doses, respectively, of CoronaVac, produced by China’s Sinovac Biotech, while Turkey will receive its first shipment of CoronaVac on 11 December. All three countries have signed contracts with for larger shipments. CanSino Biologics has a deal to sell 35mn doses to Mexico, financed by a Chinese loan. China National Biotec Group, a unit of state-owned Sinopharm, is conducting trials for two vaccine candidates in at least ten countries, which are likely to purchase the vaccine in bulk once its efficacy is demonstrated.
China's cooperation with foreign countries on vaccine trials was both a diplomatic gesture and a practical necessity. Successful containment of the virus within China meant that the country was ill-suited for large-scale clinical trials. But China has already vaccinated around 1mn military service people and civilians who travel abroad – including construction workers, diplomats, and students – outside clinical trials under an emergency use order.
Under President Donald Trump, the US has adopted an "America first" approach, striking deals with Western pharmaceutical groups to obtain vaccines for domestic use but offering no significant assistance to other countries. While the incoming Biden administration will avoid "America first" rhetoric, the severity of the US outbreak will make it politically perilous for President-Elect Joe Biden to prioritize foreigners' needs over those of Americans.
Still, Chinese firms will have to overcome skepticism about the safety and efficacy of their vaccines. None of the Chinese companies developing vaccines have yet released clinical data from phase 3 trials. But at least one technical factor favors Chinese vaccines: unlike candidates from Pfizer and Moderna, at least three of the Chinese vaccine candidates need not be stored at freezing temperature, which would reduce complications for transport and distribution. Like the Chinese vaccines, AstraZeneca's candidate may also be able to be stored safely at cold but not freezing temperatures.
Overall, the outlook for China's vaccine diplomacy highlights a growing divide between developing and developed countries in terms of the impact of the pandemic on China's international standing. For the former group, China's medical diplomacy has often been poorly received, exacerbating pre-pandemic concerns about China's rising global influence. Chinese demands for public expressions of gratitude for PPE shipments, early quality problems with some Chinese PPE, and alleged Chinese disinformation about the origin of the virus sparked resentment in the US, Western Europe, and Japan. But much of the developing world sees China as a lifeline and is willing to overlook ham-handed tactics.